Can a cloned rotavirus shell make for a more effective vaccine?
|

Can non-living biological replicas help protect children from severe diarrheal disease? Sounds eerie, but it’s a key question for Dr. Khuzwayo Jere, a Malawi-based Medical Virologist and Wellcome Trust Research Fellow, whose work on creating combined, or “chimeric”, virus-like particles may help inform next generations of rotavirus vaccines.
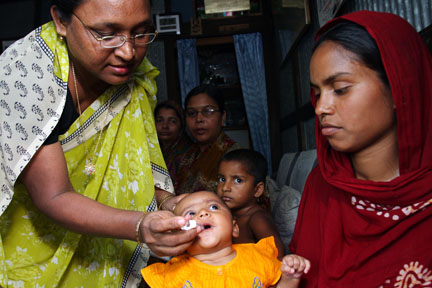
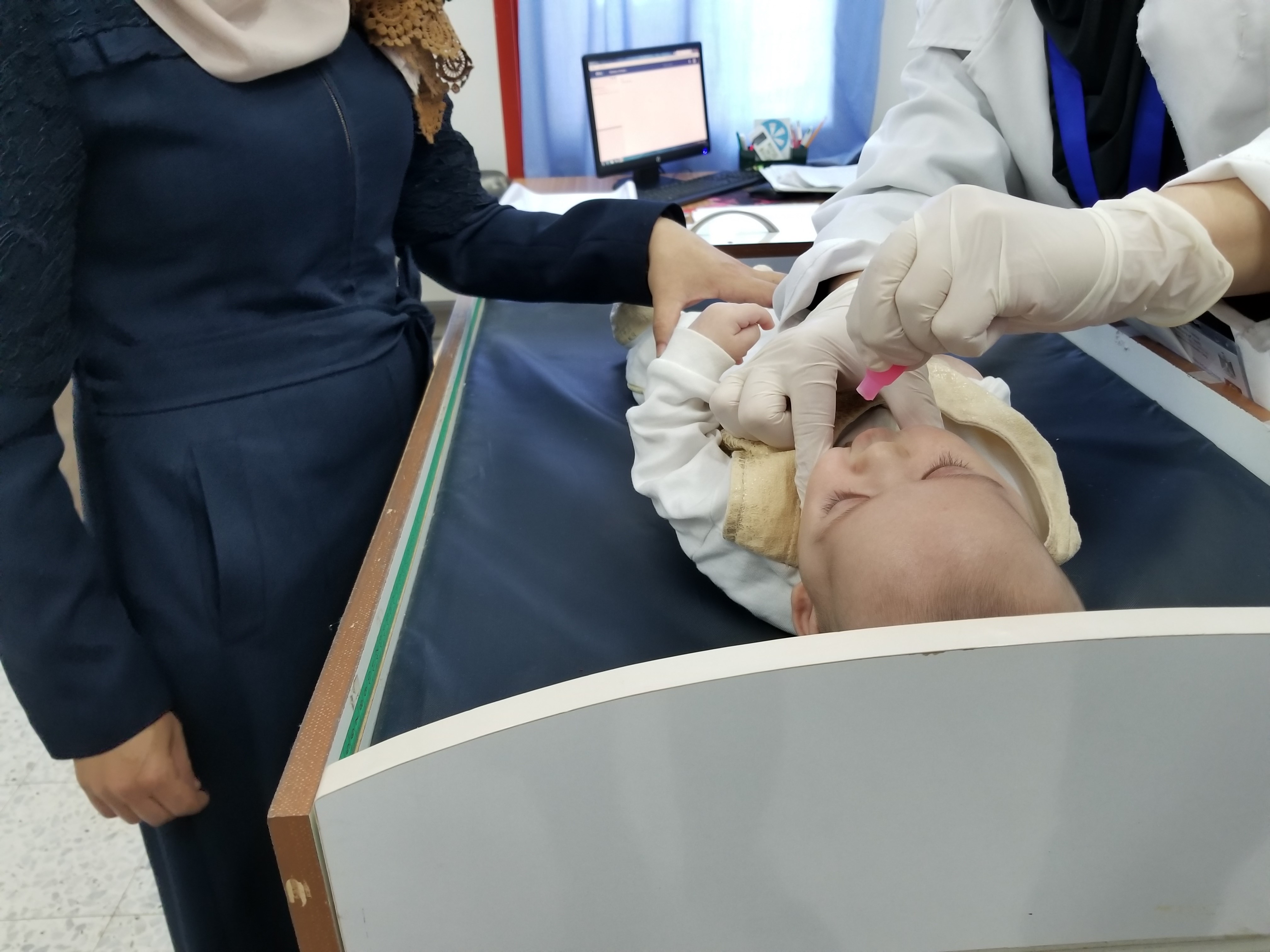
Rotavirus is the leading global cause of severe diarrhea in young children, killing about 200,000 children each year, primarily in poor countries. Rotavirus mortality has dropped considerably following the introduction of rotavirus vaccines in nearly 100 countries over the past decade. The vaccine currently used in Malawi, ROTARIX, is only moderately effective in high-burden, low-income countries when compared to high-income countries. This could be due to several factors—and one hypothesis is strain diversity.
While eight rotavirus strains account for most disease in humans, over 70 have been identified. Where transmission is high, children are frequently infected with non-vaccine strains. In Malawi, only 35% of rotavirus infections are caused by the viral strain used in ROTARIX, though there is evidence that the vaccine does protect children against a wide range of rotavirus strains. Nonetheless, developing a vaccine that protects against many strains continues to be an area of interest for rotavirus vaccine research.
All rotavirus strains have two outer capsid proteins, which are the primary vaccine targets for generating immune response. With this in mind, Dr. Jere used cloning to create chimeric virus-like rotavirus particles—empty shells constructed from viral proteins, which stimulate the immune system, just like the real virus. In a series of experiments, Dr. Jere amplified the genes of rotavirus’ four capsid proteins using virus obtained from stool samples of Malawian patients. Using a process called consensus sequencing, he narrowed the number of protein clone options while still adequately representing the genetic diversity of rotavirus in this population.
Selected rotavirus protein genes were inserted into an insect virus genome to create new, combined viruses, which were then infected into insect cell lines. The result: single-cell factories that churn out a diversity of cloned caspid proteins. Dr. Jere used electrophoresis and Western Blot as microscopic rulers to determine if the cloned proteins were identical in size and shape to the real thing.
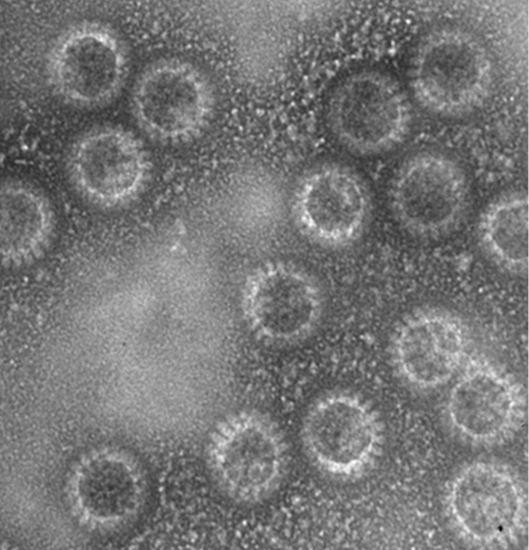
But how are virus-like particles constructed from the cloned proteins? Turns out, it’s easy. As Dr. Jere explains, “…the rotavirus capsid proteins co-assemble by themselves. No one really knows how they do it.” Once self-assembled, virus-like particles look like real viruses – even to the immune system - but lack genomic material and therefore are non-infectious. Because the particles represent many strains, a vaccine made from them should offer broad protection against rotavirus. Remembering his experiment, Dr. Jere recalls, “When we saw the particles – that was the happiest moment of my life …in terms of science.”
Genetic engineering of rotavirus has been done since the 1980s, but Dr. Jere’s work pushes the field forward. Prior experiments used genes derived from just a few laboratory strains. But because Dr. Jere’s virus-like particles were developed from clinical stool samples in Malawi, they mirror rotavirus strain diversity in the population where the vaccine will be used. Dr. Jere’s experiments are currently being optimized in bacterial and yeast cloning systems, which are even more efficient than insect cell lines.
Virus-like particles are like zombies—undead versions of the virus—but unlike zombies, they cause no harm. Instead, they can train our bodies to recognize the threat of the real thing across a broad range of strains—and may help move us forward to a new generation of rotavirus vaccines.
Read more about Dr. Jere’s research on virus-like particles here and about his other research on rotavirus antibodies here.