
India’s rotavirus vaccines are going global
What’s better than one rotavirus vaccine? Four rotavirus vaccines.
Having a robust and diverse global market of options for vaccines is beneficial in several ways – it lowers prices, stabilizes supply, gives countries more choices, and, ultimately, increases access to save more lives. In 2018, the rotavirus vaccine market made several leaps forward with not just one, but two additional vaccines receiving World Health Organization (WHO) prequalification, a stamp of approval that opens the door for global procurement of the vaccines through United Nations agencies and Gavi, the Vaccine Alliance. Previously, GlaxoSmithKline’s ROTARIX® and Merck’s RotaTeq® were the only two WHO-prequalified options, but 2018’s advancements brought the total to four.
 The World Health Organization has prequalified four rotavirus vaccines for global use. PATHBoth of the newly prequalified rotavirus vaccines are made in India: ROTAVAC®, manufactured by Bharat Biotech, and ROTASIIL®, manufactured by the Serum Institute of India. While both are already in use in India’s Universal Immunization Programme, the WHO prequalification paved the way for their use in other countries—a major opportunity for increasing access in low- and middle-income countries that are most in need of protection from deadly rotavirus diarrhea.
The World Health Organization has prequalified four rotavirus vaccines for global use. PATHBoth of the newly prequalified rotavirus vaccines are made in India: ROTAVAC®, manufactured by Bharat Biotech, and ROTASIIL®, manufactured by the Serum Institute of India. While both are already in use in India’s Universal Immunization Programme, the WHO prequalification paved the way for their use in other countries—a major opportunity for increasing access in low- and middle-income countries that are most in need of protection from deadly rotavirus diarrhea.
In 2019, this opportunity is now in motion. Palestine became the first setting outside of India to use ROTAVAC in routine immunization after the Palestinian Ministry of Health (MOH) completed its transition from ROTARIX to ROTAVAC in October 2018. Several other countries—both Gavi and non-Gavi—are planning to introduce or switch to ROTAVAC or ROTASIIL in 2019 or 2020.
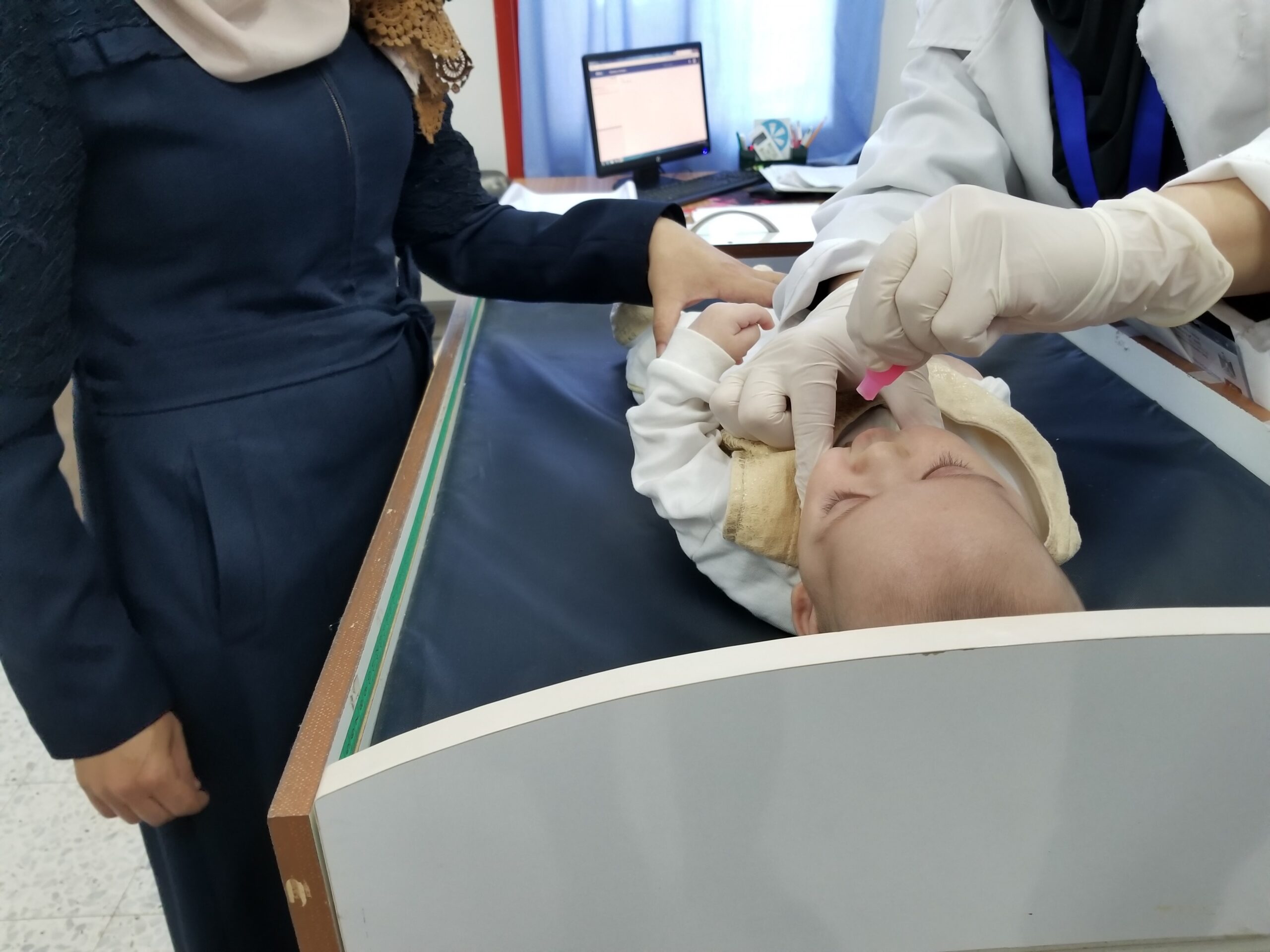
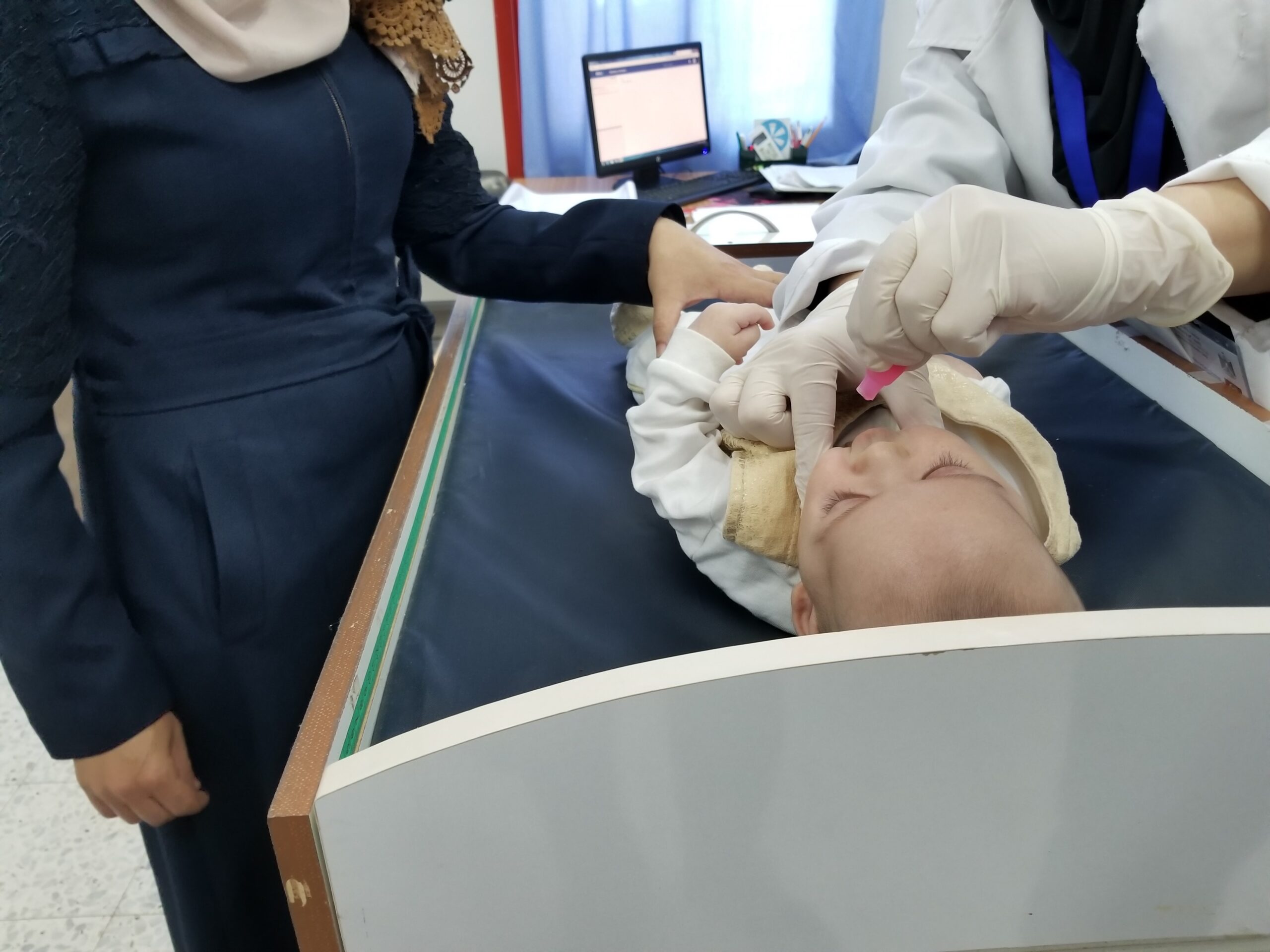 ROTAVAC®, a newly prequalified rotavirus vaccine from India, was introduced in Palestine’s routine immunization program in fall 2018. Niranjan Bhat/PATHBehind all vaccine introductions and switches, however, lies a difficult process: decision-making. Country decision-makers in ministries of health and finance must first decide to introduce a vaccine, then choose which specific product to use, and finally, plan ahead for costs, logistics, and cold chain space. In order to help countries decide which vaccine is best suited for their national immunization programs, PATH is working with several partners to collect and generate new evidence on projected costs and health and programmatic impacts of all available rotavirus vaccines.
ROTAVAC®, a newly prequalified rotavirus vaccine from India, was introduced in Palestine’s routine immunization program in fall 2018. Niranjan Bhat/PATHBehind all vaccine introductions and switches, however, lies a difficult process: decision-making. Country decision-makers in ministries of health and finance must first decide to introduce a vaccine, then choose which specific product to use, and finally, plan ahead for costs, logistics, and cold chain space. In order to help countries decide which vaccine is best suited for their national immunization programs, PATH is working with several partners to collect and generate new evidence on projected costs and health and programmatic impacts of all available rotavirus vaccines.
For instance, PATH is working in Palestine with the RVF (Rostropovich-Vishnevskaya Foundation) to support the Palestinian MOH in evaluating the impact of the switch. The study team is collecting data to estimate the 10-year costs, health impacts, and cost-effectiveness of the rotavirus vaccination program using either or both ROTARIX and ROTAVAC. This analysis will help inform the global community and other countries considering a switch.
In Zambia, PATH is working with the Centre for Infectious Disease Research-Zambia to evaluate and compare the safety, reactogenicity, and immunogenicity of the currently prequalified frozen formulation of ROTAVAC and a new liquid formulation of the vaccine. Both formulations have been shown to be safe and effective in India, and it will be important to demonstrate their performance in other settings, including Africa, in order to aid with decision-making in diverse locations. The trial launched in January, marking it the first use of ROTAVAC in an African setting.
 A Zambian infant receives rotavirus vaccine as part of a clinical trial of ROTAVAC in Zambia, January 2019. Daniel Banda/CIDRZPATH is also helping numerous countries model and evaluate the potential cost-effectiveness of different rotavirus vaccines using existing public health data, including those countries no longer eligible for Gavi support. For instance, a recent analysis from Mongolia estimated that ROTARIX, RotaTeq, or ROTAVAC would be cost-effective, but that in the absence of Gavi funding, a rotavirus vaccination program using ROTAVAC would be the most affordable out of the three.
A Zambian infant receives rotavirus vaccine as part of a clinical trial of ROTAVAC in Zambia, January 2019. Daniel Banda/CIDRZPATH is also helping numerous countries model and evaluate the potential cost-effectiveness of different rotavirus vaccines using existing public health data, including those countries no longer eligible for Gavi support. For instance, a recent analysis from Mongolia estimated that ROTARIX, RotaTeq, or ROTAVAC would be cost-effective, but that in the absence of Gavi funding, a rotavirus vaccination program using ROTAVAC would be the most affordable out of the three.
With increasing vaccine market diversity and informed decision-making, countries will be better able to access, afford, and distribute rotavirus vaccines to save the most lives from deadly rotavirus diarrhea. Thanks to two new vaccines from India, this is now becoming a reality. As additional vaccine products and presentations continue to come on the market, rotavirus deaths could one day become a thing of the past.


