Making (rotavirus) history with the launch of a new study on a novel vaccine
|

While surely all researchers are excited when they initiate a new clinical trial, we are feeling particularly energized about a Phase 3 study that we started here in Zambia this week. It is the first efficacy study for a new type of rotavirus vaccine, and we are the first of several countries that will take part in this coordinated global trial. It’s a rare privilege to be involved in a Phase 3, multi-country trial for such a groundbreaking approach to preventing a significant cause of childhood mortality like rotavirus. We are delighted to be part of the vaccine development process for this candidate.
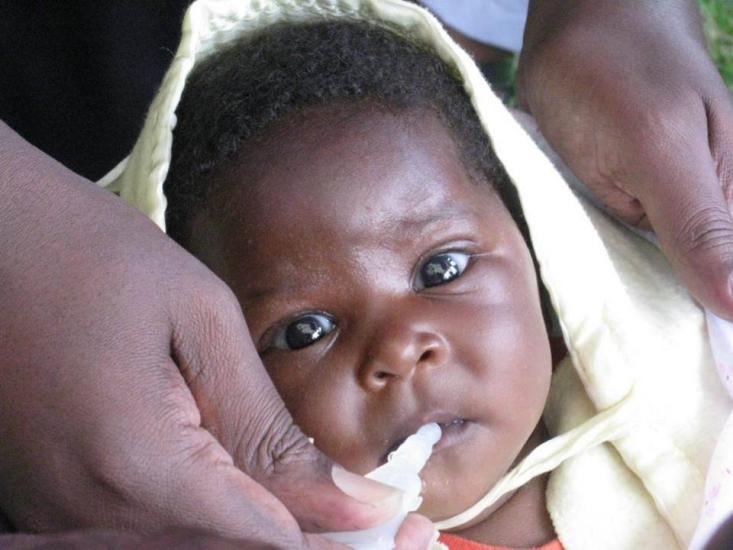
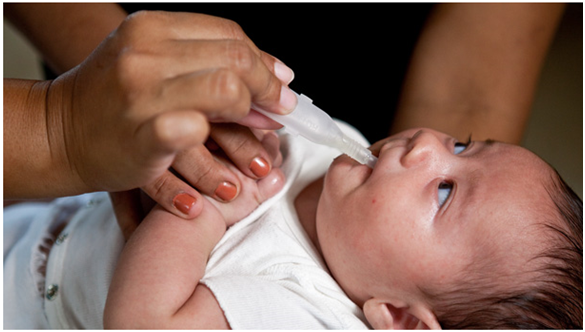
Vaccination offers the best protection for infants against severe diarrhea and death from rotavirus infection. Currently, four live, orally administered rotavirus vaccines are available for global use. While it’s clear that these vaccines are saving lives and reducing disease, we’re always striving to do even better. In places where rotavirus burden is highest, these vaccines prevent illness only half of the time. Rotavirus remains a leading cause of diarrheal disease in infants.
Non-replicating rotavirus vaccines (NRRVs) represent a new approach. These vaccines are injected into the muscle and only contain small pieces of rotavirus, which enable the body to develop an immune response. Because they are injected and bypass the gut, NRRVs may overcome factors believed to be responsible for the lower efficacy of the current oral vaccines in high-burden settings. This could potentially provide superior protection against disease.
The NRRV candidate we’re evaluating, called trivalent P2-VP8, contains tiny pieces of the three most common rotavirus strains worldwide, which we expect will result in broad protection against disease. This vaccine is also expected to be even more affordable than the currently available oral rotavirus vaccines. Affordability could facilitate uptake in countries with the highest burden—like Zambia—and free up funds for other health priorities.
We are working in collaboration with PATH, along with the other sites in Ghana and Malawi, to conduct the study. It’s designed to evaluate the safety, immunogenicity (the body’s ability to induce an immune response against rotavirus infection), and efficacy of three doses of trivalent P2-VP8 relative to a licensed, live oral vaccine (ROTARIX®) in preventing severe rotavirus among healthy infants. Following an interim analysis to get an early read on how well the study vaccine is working, enrollment will be expanded to include three additional study sites in India. In total, we will enroll approximately 8,200 healthy infants 6 to 8 weeks of age across all of the sites in Africa and Asia.
This pivotal study will provide key data on vaccine efficacy and safety in parts of the world where rotavirus has the largest burden. While we still have a long journey ahead—the vaccine wouldn’t be available for global markets until about 2025—this large-scale trial will surely be an interesting one to follow. If this innovative vaccine is successful, it has the potential to save many more lives and become a promising addition to the global rotavirus vaccine portfolio.