
Myanmar’s rotavirus vaccine campaign first to use new delivery technology

Photo: PATH/Minzayar.
This February, the Myanmar Ministry of Health and Sports—with support from the United Nations Children’s Fund and Gavi, the Vaccine Alliance—launched the first rotavirus immunization program in the country. Rotarix®, manufactured by GlaxoSmithKline (GSK), will be incorporated into Myanmar’s routine immunization program for children under five years old to prevent the incidence of rotavirus. Rotarix is a World Health Organization–prequalified oral vaccine currently in use in 96 countries.
The Myanmar launch of Rotarix marks the first time that a blow-fill-seal (BFS) container is being used to administer the vaccine. This new presentation is multi-monodose: five single doses in attached squeezable tubes that separate for easy oral delivery.
Globally, rotavirus infection is the leading cause of child morbidity and mortality from diarrheal disease—one of the top five causes of death in children under the age of five.
In Myanmar, diarrhea is among the prioritized childhood diseases included in the 2017–2021 National Health Plan, where rotavirus is the leading cause of severe acute gastroenteritis in children under five and responsible for half of the hospitalizations of that age group from 2009 through 2014.
Improving access to the vaccine for children in low- and middle-income countries at risk of gastroenteritis from rotavirus can reduce hospitalizations and mortality and improve children’s quality of life. But because the rotavirus vaccine requires a large dose volume, conventional vaccine squeeze tubes are bulky and difficult for immunization programs to transport and store.
With the aim of developing a more compact container that could enable more children to be reached with this lifesaving vaccine, researchers at PATH developed a multi-monodose BFS container design compatible with oral rotavirus vaccine. PATH generated information demonstrating the potential cost and cold chain savings associated with the BFS container, evaluated the acceptability and applicability of this new approach, and broadly shared their concepts to inform vaccine manufacturers interested in adopting this filling method.
The use of BFS for Rotarix vaccine is expected to reduce cold chain volumes by approximately 30 percent.
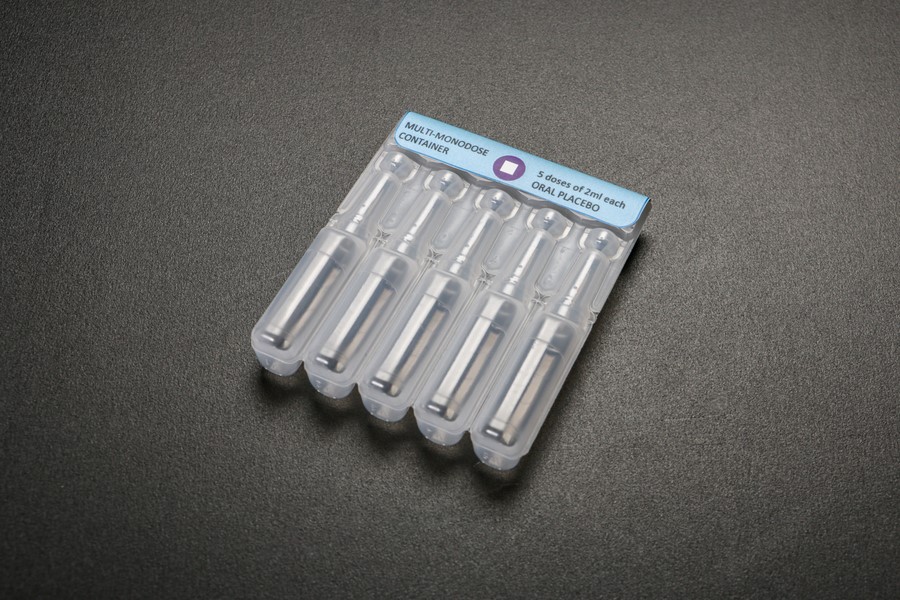 An early prototype of a blow-fill-seal container. The multi-monodose BFS packaging requires only one vaccine vial monitor label to help health workers detect heat damage, rather than a separate label for each single dose. Photo: PATH/Patrick McKern.BFS uses a polymer material to form single-dose containers for vaccines and pharmaceuticals. By using a blow molding technique combined with aseptic filling, BFS technology allows manufacture, filling, and sealing to be integrated into a single production line. This process differs from other filling techniques in that it does not require sterile containers to be produced and shipped to pharmaceutical manufacturers for filling and finishing, which improves safety and efficiency. The BFS filling method is widely used for packaging other pharmaceuticals, but has not previously been applied to vaccines. BFS containers can be formed in a vast variety of shapes and sizes, enabling designs to be tailored to meet the needs of each vaccine and optimize ease of use, minimize size, and reduce waste.
An early prototype of a blow-fill-seal container. The multi-monodose BFS packaging requires only one vaccine vial monitor label to help health workers detect heat damage, rather than a separate label for each single dose. Photo: PATH/Patrick McKern.BFS uses a polymer material to form single-dose containers for vaccines and pharmaceuticals. By using a blow molding technique combined with aseptic filling, BFS technology allows manufacture, filling, and sealing to be integrated into a single production line. This process differs from other filling techniques in that it does not require sterile containers to be produced and shipped to pharmaceutical manufacturers for filling and finishing, which improves safety and efficiency. The BFS filling method is widely used for packaging other pharmaceuticals, but has not previously been applied to vaccines. BFS containers can be formed in a vast variety of shapes and sizes, enabling designs to be tailored to meet the needs of each vaccine and optimize ease of use, minimize size, and reduce waste.
In the future, BFS technology may also be used to package other vaccine products and could potentially simplify delivery and reduce the cold chain volumes of other oral and injectable vaccines. These types of innovations in vaccine technology will play a critical role in reaching children in low- and middle-income countries to reduce the incidence of rotavirus and other infections.


