Maximizing the use and impact of rotavirus vaccines: PATH’s priority
|
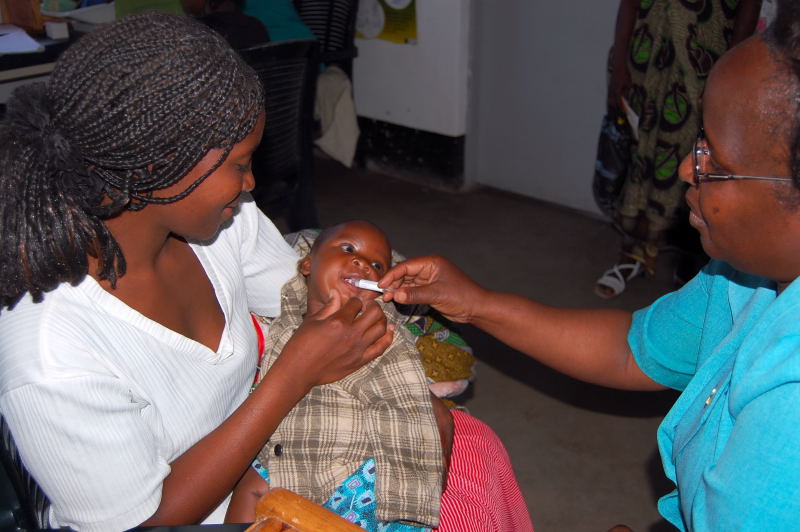
You probably already know the shocking reality—more than 1,200 children under age five die from rotavirus each day—that's more that 450,000 each year! What you might not know is that many of these deaths can be prevented by using rotavirus vaccines. Vaccination offers the best protection against severe rotavirus diarrhea, and rotavirus vaccines are saving lives in countries where they are in use.
This is why I am so excited about important news in “Rotavirus Vaccines for Children in Developing Countries”, a special supplement to the journal Vaccine, published this week. The supplement analyzes evidence from scientific studies that examine how rotavirus vaccines work in developing countries that contribute to 95% of the global death toll from rotavirus. The studies demonstrate that rotavirus vaccines are safe, proven, and cost-effective and are projected to save more than 2.4 million lives by 2030 in developing countries. The supplement also reports that rotavirus vaccines significantly reduce serious rotavirus disease in rural settings, where children often die from rotavirus infection because their mothers can't reach medical care in time to access lifesaving rehydration treatment for their infants.
PATH has a longstanding commitment to maximizing the impact of rotavirus vaccines in low-resource settings and accelerating their access to children most in need. Just five years ago, the World Health Organization (WHO) lacked sufficient scientific evidence about rotavirus vaccines to recommend their global use. PATH's Rotavirus Vaccine Program (RVP), a partnership between PATH, the Centers for Disease Control and Prevention, and WHO, funded by the GAVI Alliance, set about to change that and bring rotavirus vaccines to the developing world—to the kids who need them most urgently. It was this mission that motivated me to join PATH after having spent my career focused on vaccine policy and performance in US populations. RVP worked with rotavirus vaccine manufacturers to conduct clinical trials in Africa and Asia to help WHO assess their effectiveness in those populations.
In June 2009, informed in large part by the clinical trials sponsored by RVP and its partners, WHO recommended that rotavirus vaccines be introduced in all countries around the world. Remarkably, just two years later, Sudan became the first African country to introduce rotavirus vaccines into its national immunization program with GAVI-support.This week, two more GAVI-eligible countries, Yemen and Ghana, will roll out rotavirus vaccines. That brings the total to seven GAVI-eligible countries to date, with several more expected to introduce rotavirus vaccines by 2013.Typically, it takes 10 to 15 years for new vaccines to reach developing world populations—this speed of introduction is unprecedented.
RVP's journey wasn't always easy—conducting rigorous scientific studies in real-world, low-resource environments was challenging. But this was where the rotavirus burden was most dire, the loss of life most acute, and the potential benefit most profound. With dedicated and inspiring partners in countries like Bangladesh, Ghana, Mali, and Malawi, the RVP team developed a deep appreciation of the realities faced by these countries.
While this supplement is RVP's culminating publication, PATH's dedication to helping children in developing countries access rotavirus vaccines and identify ways to enhance rotavirus vaccine performance in low-resource settings is unwavering. PATH continues RVP's work as part of the GAVI-supported Accelerated Vaccine Introduction initiative Technical Assistance Consortium (AVI TAC) and the Bill & Melinda Gates Foundation-supported Rotavirus Vaccine Impact (RVI) project. In addition, PATH is collaborating with emerging-country manufacturers to advance new rotavirus vaccines for the developing world. During RVP's tenure, I served as Clinical Director, and I am looking forward to continuing to support PATH's rotavirus work under AVI TAC and RVI. It is an exciting time to be working in the field of rotavirus vaccines!
-- Dr. Kathy Neuzil is the incoming Director of the Vaccine Access and Delivery Global Program at PATH and co-editor of the “Rotavirus Vaccines for Children in Developing Countries” special supplement to the journal Vaccine.
For more information:
-- This week is the World Health Organization's first ever World Immunisation Week. To celebrate, Ghana will be the first country to simultaneously introduce vaccines against its two biggest killers: pneumonia and rotavirus. Follow the conversation on Twitter with the hashtag #vaccineswork.