ROTAVIRUS
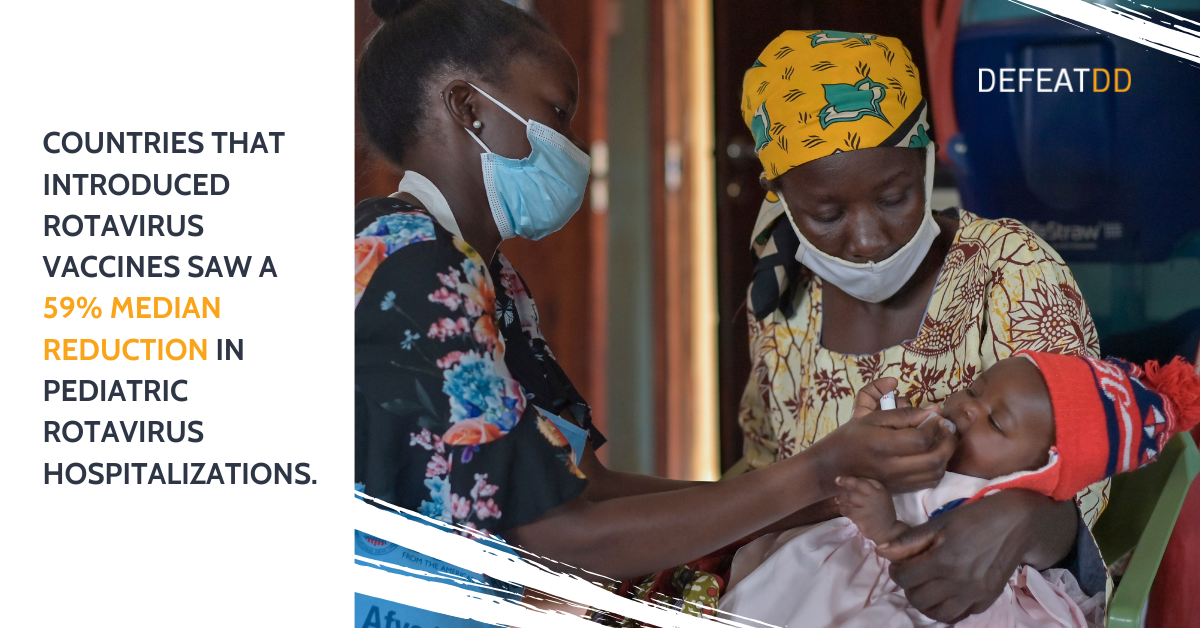
Rotavirus is the leading cause of severe childhood diarrhea. It disproportionately burdens impoverished communities of Africa and Southeast Asia. But nearly every child in the world is at risk. Rotavirus infections cannot be treated with antibiotics or other drugs, making vaccines a vital solution for preventing childhood illness and death. The World Health Organization recommends rotavirus vaccines for all countries.
For two decades, PATH has worked to ensure that all children have access to rotavirus vaccines. We work with manufacturers to develop low-cost vaccine products and sustain global supply. In partnership with country decision-makers, we help to assess, evaluate, prepare for, and optimize rotavirus vaccine introduction.