Confirmed: Ghana’s switch to ROTAVAC was cost-saving

The PATH Ghana team hosted a meeting in Accra on May 24, 2022 to share the results of the ROTAVAC switch costing study with interested stakeholders and partners. Photo: PATH/Patience Dapaah.
Editor’s note: The manuscript for this study is now available here.
In 2020, despite complications from the COVID-19 pandemic, Ghana switched from using ROTARIX® to ROTAVAC® rotavirus vaccine in its national immunization program. The Ghana Health Service made this decision for two main reasons: to save money, increasing the sustainability of its immunization program and decreasing Gavi support, and to save cold chain space. Previous economic analyses suggested these goals could be reached, but actual experience can differ from projections. Because several countries are currently undergoing or considering switching rotavirus vaccine products, Ghana’s switch was poised to serve as a useful case study to assess the costs and implications of a switch.
For the last two years, PATH worked with Ghana Health Service and the University of Ghana to analyze the economic implications of this switch. As the team lead for Vaccine Implementation, Africa in PATH’s Ghana office and part of the study team for this analysis, I have been eager to see the results. With the analysis now complete, we can finally confirm: Ghana’s switch to ROTAVAC successfully accomplished its goals.
A smooth transition
Switching from one vaccine product to another requires a range of activities. 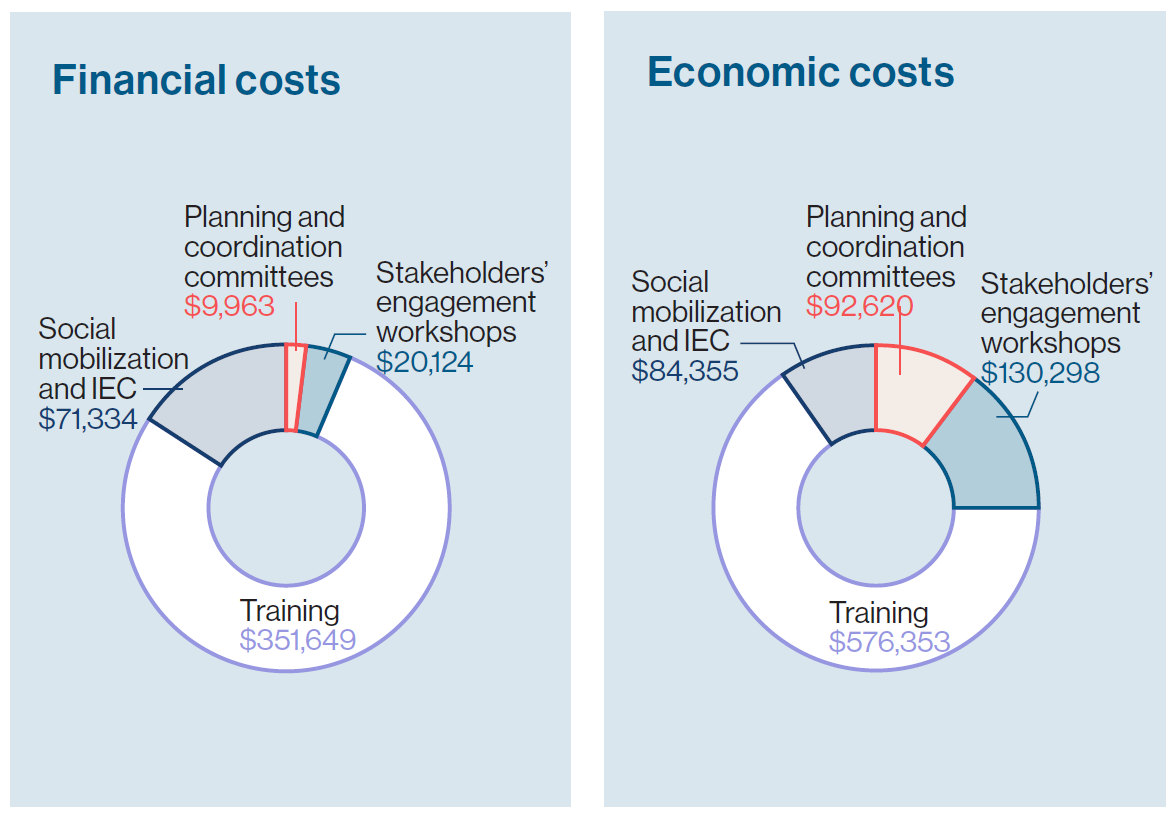 For Ghana, this included planning meetings, training sessions at the central, regional, district, and health facility levels, and social mobilization and information, education, and communication (IEC) activities. Such a switch might usually require procurement of refrigerators, transportation, and other logistics resources, but in this case, minimal costs were incurred for vaccine storage, transport, or service provision because of the switch. In fact, only one district in the study sample reported that a refrigerator had been provided to a facility.
For Ghana, this included planning meetings, training sessions at the central, regional, district, and health facility levels, and social mobilization and information, education, and communication (IEC) activities. Such a switch might usually require procurement of refrigerators, transportation, and other logistics resources, but in this case, minimal costs were incurred for vaccine storage, transport, or service provision because of the switch. In fact, only one district in the study sample reported that a refrigerator had been provided to a facility.
After assessing all these switch activities and associated costs, the analysis determined that the rotavirus vaccine switch required $453,070 in financial expenditures or a total of $883,626 in economic costs—which includes expenditures plus the value of in-kind resources used, such as salaries of existing healthcare staff. The biggest driver for the switch costs was training (77.6% of expenditures) followed by social mobilization and IEC activities (15.7%).
Overall, the transition was smooth, required minimal costs, and caused no interruptions in rotavirus vaccination coverage.
ROTAVAC: A cost-saving solution
Accounting for the cost of switching as well as the difference in costs of procuring and delivering ROTAVAC compared to ROTARIX, the analysis found that switching to ROTAVAC—using either the 5-dose vial or 10-dose vial—was cost-saving for Ghana. This is great news for the Ghana Health Service, as it validates its decision to switch.
The analysis found that using ROTAVAC will save the government approximately $20 million in vaccine procurement costs over the next decade, even after the country stops receiving Gavi support, which is currently projected for 2027. While the supply chain and delivery costs per course were higher for ROTAVAC due to its third dose, the lower cost of the vaccine outweighs these costs, resulting in a net $10 million in savings for Ghana over 10 years.
Reduced cold chain requirements
Finally, the analysis looked at the change in cold chain requirements for ROTAVAC compared to ROTARIX. Even though ROTAVAC requires three doses and ROTARIX only requires two, ROTAVAC has a considerably smaller cold chain requirement per course compared to ROTARIX. Overall, the required cold chain volume for a full 3-dose course of ROTAVAC is about one-third of the cold chain volume needed for a full course of ROTARIX.
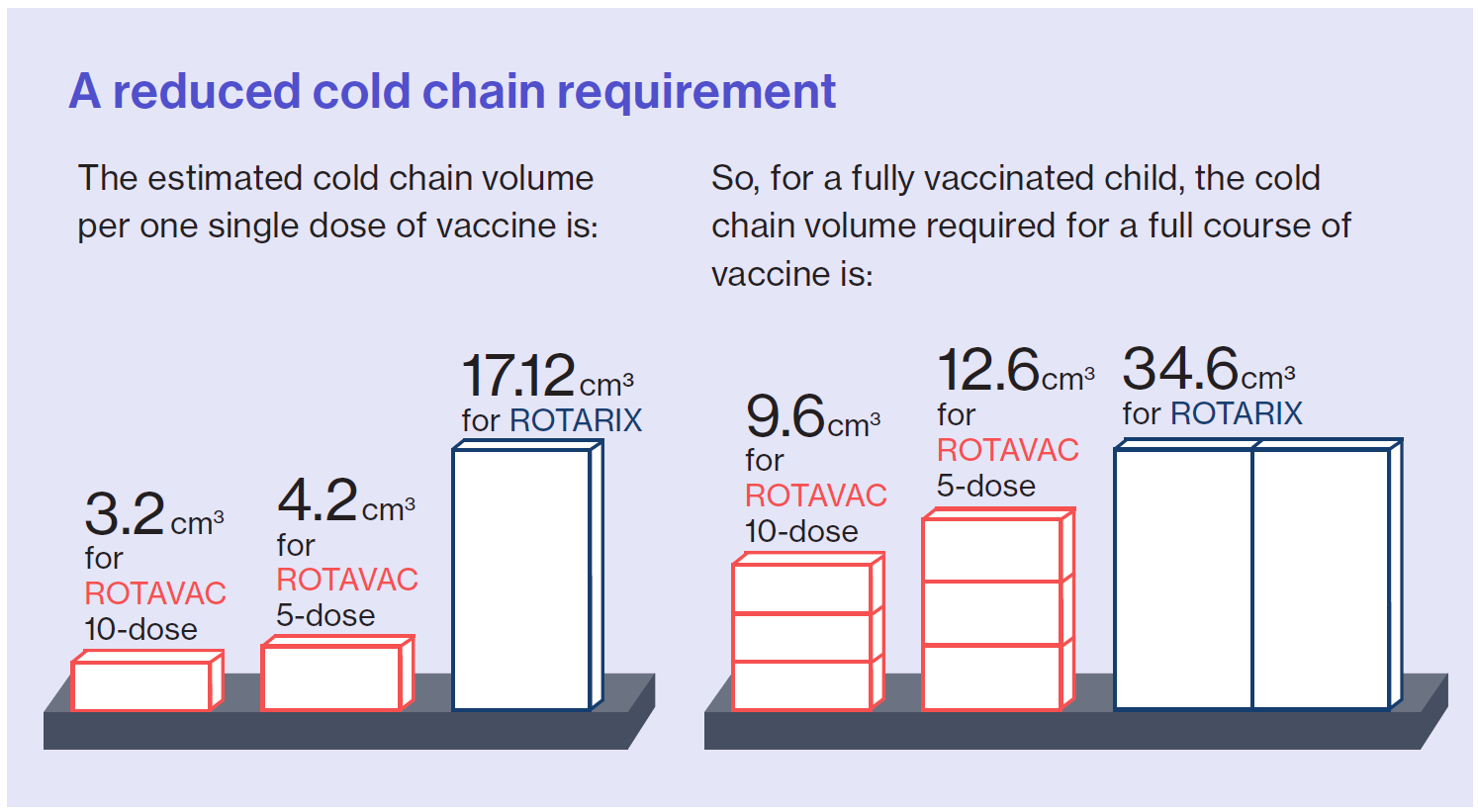
Additionally, while the Ministry of Health originally switched to the 5-dose vial presentation of ROTAVAC, they are now considering the 10-dose presentation due to its further reduced cost and even lower cold chain volume per course.
Sharing Ghana’s findings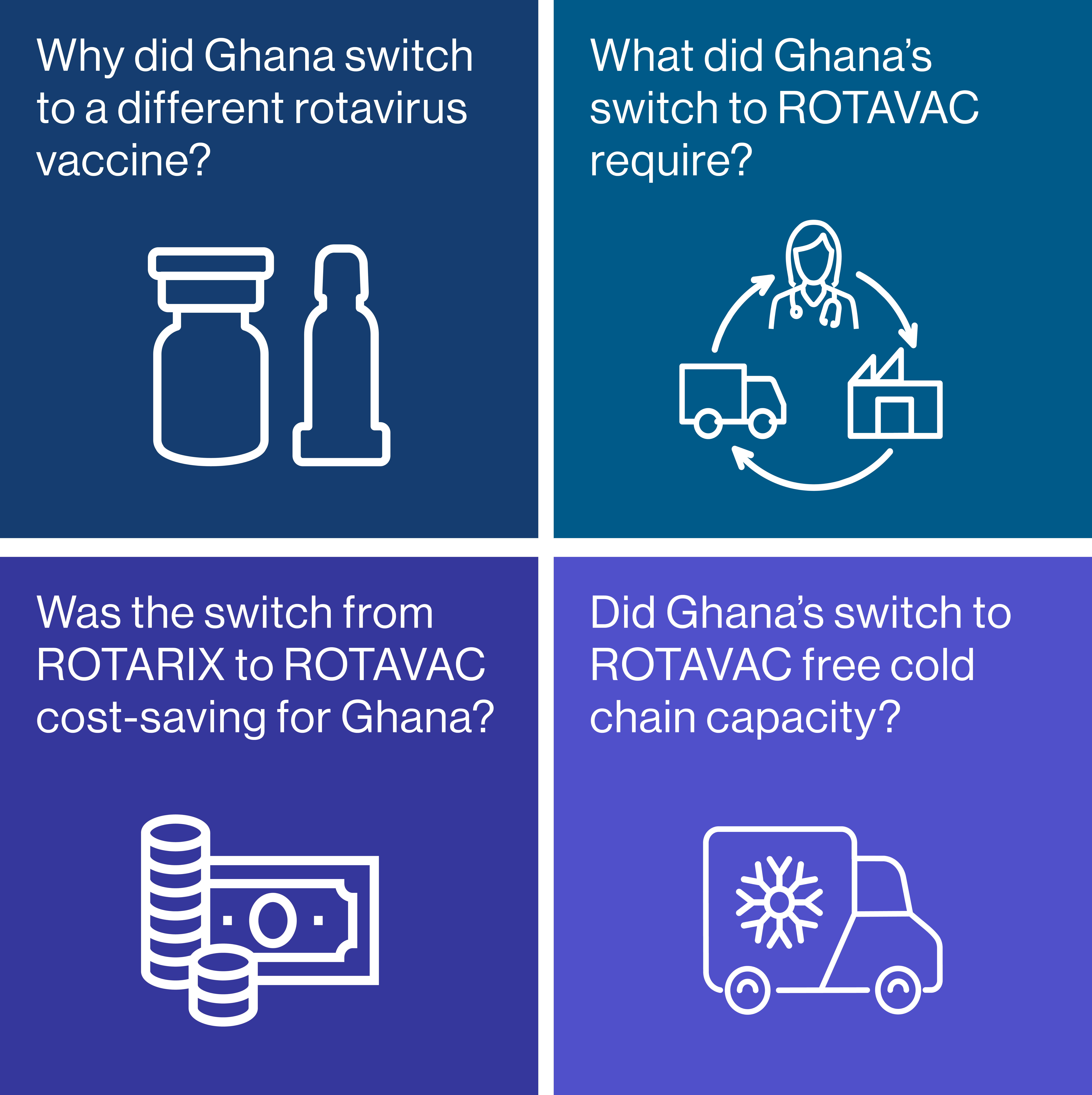
We believe this new evidence on the related costs and cost-effectiveness of changing vaccine products in Ghana will be helpful to inform other countries in similar situations. The availability of new, lower-cost rotavirus products and the increasing number of countries transitioning out of Gavi support may prompt many countries to switch, and the example of Ghana should provide insightful lessons. To share these lessons with other countries and stakeholders, check out our case study answering four key questions from the economic analysis of Ghana’s switch from ROTARIX to ROTAVAC, available here.
Additionally, we recently held a meeting in Ghana to share the findings with national partners and stakeholders, who were relieved to hear the results. Confirmation that a policy decision had its intended benefit is a great reassurance. We hope that this confirmation will help decision-makers in Ghana and around the world to listen and act on the evidence.